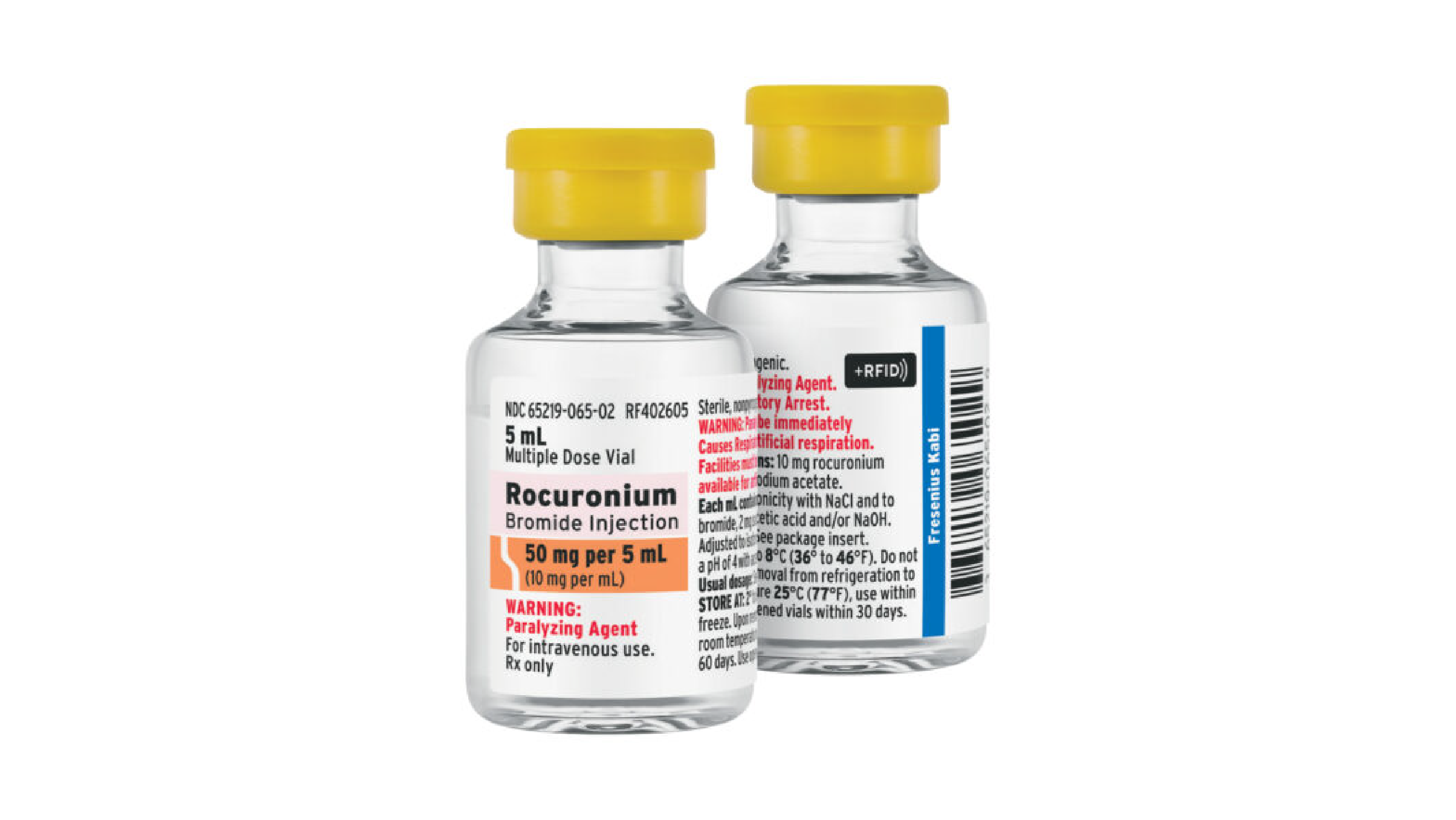
Fresenius Kabi’s RFID pre-tagged Rocuronium Bromide Injection is fully compatible with all major RFID kit and tray vendors
ALEXANDRIA, VA, May 15, 2023 – Fresenius Kabi, a leading provider of injectable medications, announced today it has launched Rocuronium Bromide Injection with advanced RFID-enabled labels in the United States. Rocuronium Bromide Injection is part of Fresenius Kabi’s +RFID™ portfolio of smart-labeled medications, designed to help enhance patient safety and support a more-efficient medication inventory process. This will mark the first +RFID product that is compatible with Bluesight’s KitCheck product, the leading kit and tray solution for American hospitals allowing +RFID products to be used across all major RFID kit and tray vendors.
The use of RFID technology with pharmaceutical products supports a safe, more efficient medication inventory process that eliminates manual product tagging, data entry and may help improve patient safety by reducing medical errors.
“We’re happy to add Fresenius Kabi to our growing community of partners committed to qualifying and testing RFID products to ensure customers will be able to use them, and we are excited for the portfolio of compatible RFID products to grow. Fresenius Kabi’s use of the registry will provide hospitals the ability to instantly identify recalls and use +RFID products across the hospital system,” said Kevin MacDonald, CEO of Bluesight.
Fresenius Kabi Rocuronium Bromide Injection +RFID is available direct from the company and U.S. wholesalers in a 50 mg per 5 mL multiple dose vial. Utilizing RAIN RFID technology, the smart-labeled medication, following GS1’s open global data, has attained ARC certification by Auburn University’s RFID lab.
According to a recent study by the ASHP Foundation, 87 percent of hospitals surveyed indicate they will evaluate or use RFID in their facility, highlighting the growing demand for RFID technology in the health care industry.1 Fresenius Kabi currently offers four +RFID medications and, to meet the growing need, plans to introduce more +RFID medications in 2023.
“Fresenius Kabi is committed to answering our customers’ call and to support them in their search for improved methods of medication management,” said John Ducker, president and CEO of Fresenius Kabi USA. “Interoperability of our +RFID medications is critical to reducing pain points in the American hospital system.”
Fresenius Kabi produces Rocuronium Bromide Injection in the United States, where the company has invested nearly $1 billion in an advanced manufacturing and distribution network dedicated to serving U.S. hospitals and health systems. To learn more about how Fresenius Kabi is strengthening America’s supply chain of care, visit “More in America.”
INDICATIONS AND USAGERocuronium Bromide Injection is a nondepolarizing neuromuscular blocking agent indicated as an adjunct to general anesthesia to facilitate both rapid sequence and routine tracheal intubation, and to provide skeletal muscle relaxation during surgery or mechanical ventilation.
IMPORTANT SAFETY INFORMATIONRocuronium Bromide Injection is for intravenous use only. This drug should only be administered by experienced clinicians or trained individuals supervised by an experienced clinician familiar with the use, actions, characteristics, and complications of neuromuscular blocking agents. Doses of Rocuronium Bromide Injection should be individualized and a peripheral nerve stimulator should be used to monitor drug effect, need for additional doses, adequacy of spontaneous recovery or antagonism, and to decrease the complications of overdosage if additional doses are administered.
Rocuronium is contraindicated in patients with hypersensitivity (e.g., anaphylaxis) to rocuronium bromide or other neuromuscular blocking agents.
Appropriate Administration and Monitoring: Use only if facilities for intubation, mechanical ventilation, oxygen therapy, and an antagonist are immediately available.
Anaphylaxis: Severe anaphylaxis has been reported. Consider cross-reactivity among neuromuscular blocking agents.
Risk of Death due to Medication Errors: Accidental administration can cause death. Store Rocuronium Bromide Injection with the cap and ferrule intact and in a manner that minimizes the possibility of selecting the wrong product.
Need for Adequate Anesthesia: Must be accompanied by adequate anesthesia or sedation.
Residual Paralysis: Consider using a reversal agent in cases where residual paralysis is more likely to occur.
Most common adverse reactions (2%) are transient hypotension and hypertension.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Succinylcholine: Use before succinylcholine has not been studied.
Nondepolarizing muscle relaxants: Interactions have been observed.
Enhanced Rocuronium Bromide Injection activity possible: When used in conjunction with Inhalation anesthetics, certain antibiotics, quinidine, magnesium, lithium, local anesthetics, procainamide.
Reduced Rocuronium Bromide Injection activity possible: When used in patients on chronic anticonvulsant therapy.
Labor and Delivery: Not recommended for rapid sequence induction in patients undergoing Cesarean section.
Pediatric Use: Onset time and duration will vary with dose, age, and anesthetic technique. Not recommended for rapid sequence intubation in pediatric patients.
This Important Safety Information does not include all the information needed to use Rocuronium Bromide Injection safely and effectively. Please see full prescribing information for Rocuronium Bromide Injection at www.fresenius-kabi.com/us.
About Fresenius KabiFresenius Kabi (www.fresenius-kabi.com/us) is a global health care company that specializes in injectable medicines, biosimilars, and technologies for infusion, transfusion, and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany. To learn about U.S. career opportunities at Fresenius Kabi, visit us at www.fresenius-kabi.com/us/join-us and follow us on LinkedIn.
About BluesightBluesight solves supply chain inefficiencies and reduces risk by providing actionable analytics for every step of the medication lifecycle. Through our suite of Medication Intelligence™ solutions, Bluesight brings simplicity, visibility, and predictability to the complex world of medication management. To date, more than 1,000 U.S. and Canadian hospitals utilize Bluesight solutions to optimize their hospital’s efficiency.
Bluesight focuses on providing comprehensive, item-level data that empowers providers to make informed, accurate decisions. These solutions include RFID-enabled inventory management, controlled substance diversion prevention software and medication purchasing optimization. To learn more about Bluesight’s suite of Medication Intelligence solutions, visit us at bluesight.com.
+RFID is a registered trademark of Fresenius Kabi USA, LLC.
References:
- Advancing Medication Safety Through Technology Innovations: Focus on Radio Frequency Identification Technology. American Society of Health-System Pharmacists Foundation. 2022. https://www.ashpfoundation.org/-/media/REF/Research/PDFs/ASHP_RFID_Report.pdf